In Re Cellect – ODP Defense Does Not Impact The Expiration Date of A Patent With Both PTA and PTE In The Absence of A Terminal Disclaimer
September 15, 2023
The Federal Circuit in In re Cellect, Appeals Nos. 2022-1293, 2022-1294, 2022-1295, 2022-1296 held that the earliest patent to expire in a series of patents subject to obviousness-type double patenting (ODP) controls, i.e., the PTA in the later to expire patents is lost and all patents are invalid for double patenting. In Cellect the relation between the patents is shown below:
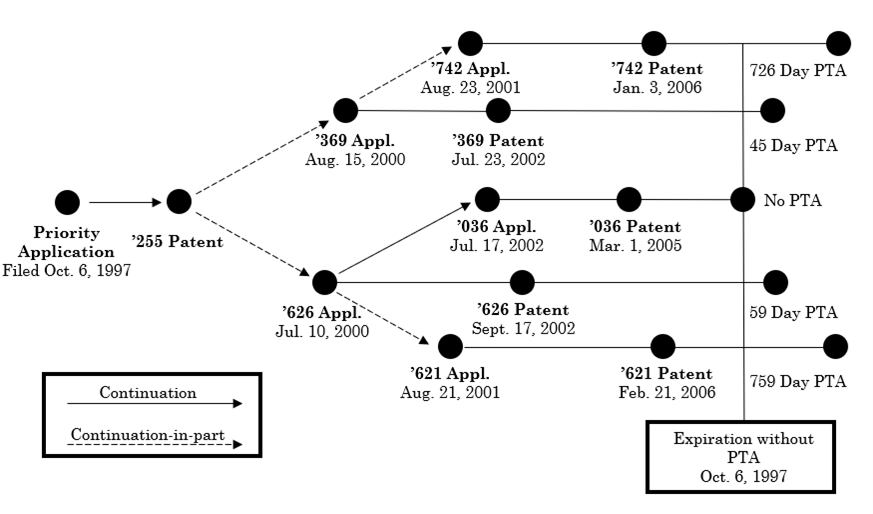
Cellect attempted to argue that the Judicial doctrine of ODP could not trump the statutory grant of PTA. This argument was rejected based on the difference in the language of 35 U.S.C. § 154, the PTA statute, and 35 U.S.C. § 156. Section 153 was silent with respect to the effect of a terminal disclaimer on PTA, while § 156 considered the impact. The Court agreed with the PTAB that this signaled that the two statutes should be treated differently for ODP purposes. Section 156 provided for PTE to be added to the expiration date of the patent even where a terminal disclaimer had been filed. No similar provision appears in § 154. The Court rejected Cellect’s argument that the USPTO’s failure to make a double patenting rejection insulated the patents from invalidation for double patenting since Cellect was not put on notice of the issue. While not mentioned in the decision, the argument could be presented for any rejection the USPTO could have made but didn’t because of examiner error, including abstract idea, 35 U.S. C. § 101 and 35 U.S.C. § 112. The failure of the USPTO to make rejections under these paragraphs does not preclude invalidation in litigation under these provisions. Cellect offered no compelling reason why § 101 should be any different.
What many of the commentators failed to note was the Court’s discussion of the interplay between PTA and PTE in a patent having both extensions and a potential double patenting issue. On pages 16 and 17 of the slip opinion the Court explained that under § 156 the PTE is to be added to the end of the PTA unless a terminal disclaimer was filed. The Court reasoned that:
In Novartis, we held that the presence of ODP would not cut off a duly granted PTE under § 156. Stated otherwise, whether or not claims are unpatentable for ODP is determined in view of the expiration date of the patents before any PTE is added. In Novartis, we merely “decline[d]” to allow “a judge-made doctrine [to] cut off a statutorily-authorized time extension.” Novartis, 909 F.3d at 1375.
Since § 156 mandates that the PTE is to be added to the end of the PTA period, the presence of possible ODP does not impact the extension calculation which would involve adding the PTE period to the end of the PTA period in the absence of a terminal disclaimer. Thus, where the USPTO errs in not making a double patenting rejection, the PTE is not affected for determining the expiration date.
A key point from Cellect is that companies with a patent forest protecting a product should review the patents for potential double patenting issues while there is an opportunity to correct it either with a terminal disclaimer or a reissue to narrow the claims to avoid the double patenting since often double patenting is caused by overly expansive claims.